Groups that are cis on the alkene will end up cis on the cyclopropane product. Other types of ylides can also react with ketones and aldehydes but alkenes are not the favored.
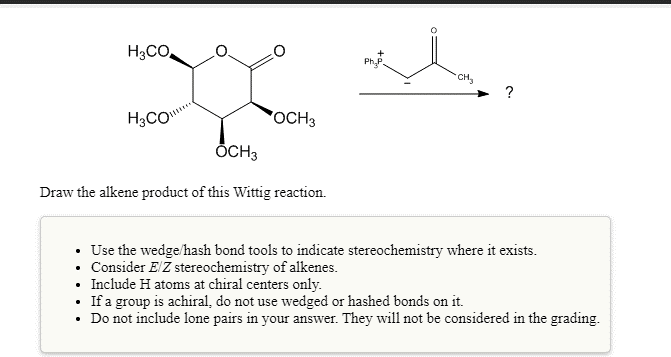
Solved H3co Php Ch H2com Och3 Och3 Draw The Alkene Chegg Com
Triphenylphosphine oxide is produced as a by-product.
. The Diels-Alder reaction for example is a 42 cycloaddition reaction four atoms from the diene and two from the dienophile. When the R group of the ylide is a simple alkyl the species is called an unstabilized ylide and the Z double bond isomer predominates in the products. Please draw the products for the following wittig reactions.
When the R group is an aryl alkenyl or. Wittig reaction is an organic chemical reaction wherein an aldehyde or a ketone is reacted with a Wittig Reagent a triphenyl phosphonium ylide to yield an alkene along with triphenylphosphine oxide. Ah and so remember the video reaction Um basically replaces a carbon deal with um whatever is attached to the phosphorus psyllid.
Um and so uh one way that we can think about this Um the way. The Wittig reaction or Wittig olefination is a chemical reaction of an aldehyde or ketone with a triphenyl phosphonium ylide often called a Wittig reagent to give an alkene and triphenylphosphine oxide. Wittig reactions are more general in that the product carbonyl does not need to have an attached carbonyl.
Water-soluble side product. The Wittig reaction was discovered in 1954 by Georg Wittig for which he was awarded the Nobel Prize in Chemistry in 1979. Adding n-butyllithium which is a base undergo deprotonation on the carbon which is connected to the PPh 3 group to get a ylide intermediate.
Indicate product and all intermediate structures for this reaction. Mechanism of the Wittig Reaction The EZ selectivity of the Wittig reaction depends upon the structure of the ylide that is used. This is a reduction.
This ylide on reaction with benzaldehyde gives an alkene. Experiment 27 A Solvent Free Wittig Reaction Page 2 of 5 Figure 3. He was also awarded the 1979 Nobel Prize in Chemistry for this.
The Wittig reaction discovered in 1954 by Georg Wittig is one of the most common tech-niques used for the stereoselective preparation of alkenes. Answer to Solved Draw the alkene product of this Wittig reaction. Wittig reactions can give either the E or Z isomer of the alkene depending on the nature of the phosphonium reagent.
The reaction of an aldehyde or ketone with a phosphonium ylide to an alkene and a phosphine oxide is known as Wittig reaction or Wittig Olefination reaction. This is the answer to Chapter 21. It is widely used in organic synthesis for.
This is known as the Wittig reaction or Wittig olefination. Another name for an alkene is an olefin. Addition of Ylides - Wittig Reaction.
Synthesis of trans-9-2-Phenylethenylanthracene In a 25-mL Erlenmeyer flask place a small stir bar 050g of 9-anthraldehyde 087g of. The alkene product 4 that you make today is the one that was used a few weeks ago as the colorizer for the chemiluminscence experiment it gave the green solution Mechanism The general mechanism of the Wittig reaction is shown above. CHEM 222 Report Sheet 2018-2019 2 Objective 3 marks.
The 22 notation is referred to the number of atoms participating in the reactionIn this it is the two atoms of the carbonyl group and the P and O from the Wittig reagent. The above reaction is known as Wittig reaction. H RR cis-alkene CH2I2 ZnCu ether H H RR cis-cyclopropane H R RH trans-alkene CH2I2 ZnCu ether H R RH trans-cyclopropane Hydrogenation.
George Wittig and his graduate students developed a very effective and operationally simple method of incorporating an alkene where once was present a ketone or aldehyde. Mechanism of the Wittig Reaction The EZ selectivity of the Wittig reaction depends upon the structure of the ylide that is used. This process is now known as th e Wittig reaction.
As above reaction easily say that which reaction are alfa decay beta decay and so on question_answer Q. In this reaction we expect primarily the E isomer. Draw the mechanism for cyclohexanone reacting with a phosphorous ylide.
A Wittig reaction involves the formation of. If a lithium base was used the intermediate β-hydroxyphosphine oxide could be isolated and transformed into the alkene in a subsequent step. When the R group on the ylide is a simple alkyl group the species is called an unstabilized ylide and the Z double bond geometry predominates.
Ylides will add to aldehydesketones and produce alkenes. The product was characterized by taking an IR spectrum and an 1H NMR. For this work George Wittig was awarded the Nobel Prize in chemistry in 1979 co-recipient HC.
The purpose of this experiment is to conduct a Wittig reaction to synthesize an alkene ethyl 4-chlorocinnamate using a 4-chlodobenzazldehyde and a stabilized ylide. The yields of di- and tri-substituted alkenes from aldehydes and ketones are very high but yields of tetra. 1913 THE WITTIG ALKENE SYNTHESIS 933 PROBLEMS 1932 Draw the structures of all aldehydes or ketones that could in principle give the following product after application of either the WolffKishner or Clemmensen reduction.
Broadly speaking the reaction allows for the formation of an alkene product and a triphenylphosphine oxide side product. Alkyl halide on reaction with PPh 3 undergo substitution reaction. The Wittig reaction also known as Wittig olefination is a great way to turn aldehydes and ketones into alkenes.
This problem asks us to draw the products of each of these too vinegary actions. Before we get into the mechanism lets look at a really quick way to get the right answer on an exam. The reaction.
PLEASE GIVE THUMBS UP. Problem number 17 fromthe Smith Organic chemistry Textbook. The 22 addition forms a four-membered ring called oxaphosphetane made.
Experiment 20 A Solvent Free Wittig Reaction Page 2 of 5 Figure 3. If a potassium base was used to generate the phosphine oxide anion the reaction with a carbonyl compound proceeded as in the Wittig reaction to give an alkene. This Reaction is named after its discoverer the German chemist Georg Wittig.
Wittig reactions are very convenient to generate an alkene starting with a ketone or aldehyde. Addition of H2 across the p-bond of an alkene to give an alkane. This reaction was discovered in 1954 by Georg Wittig for which he was awarded the Nobel Prize in Chemistry in 1979.
1933 Outline a synthesis of 14-dimethoxy-2-propylbenzene from hydroquinone p-hydroxyphenol and any other reagents.

Solved Ph3p Draw The Alkene Product Of This Wittig Reaction Chegg Com
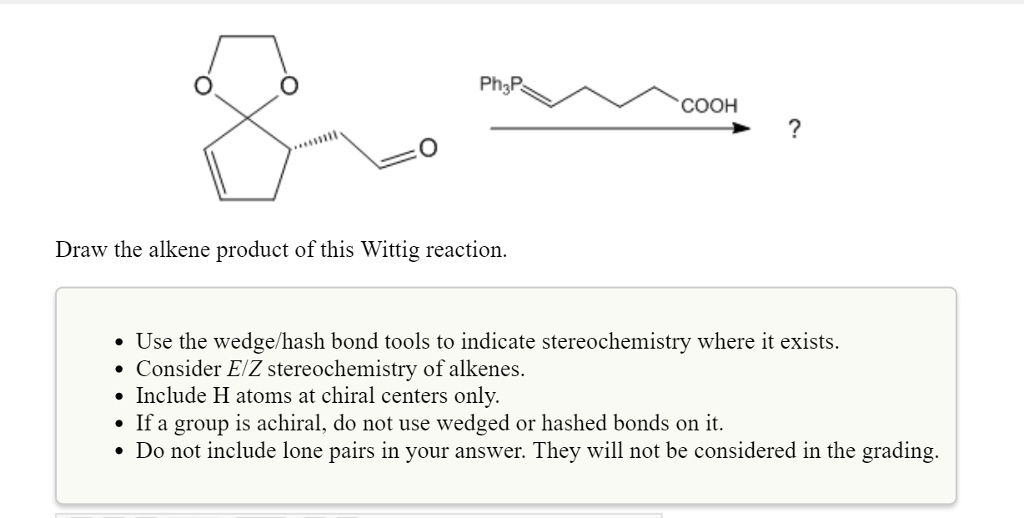
Solved Draw The Alkene Product Of This Wittig Reaction Chegg Com
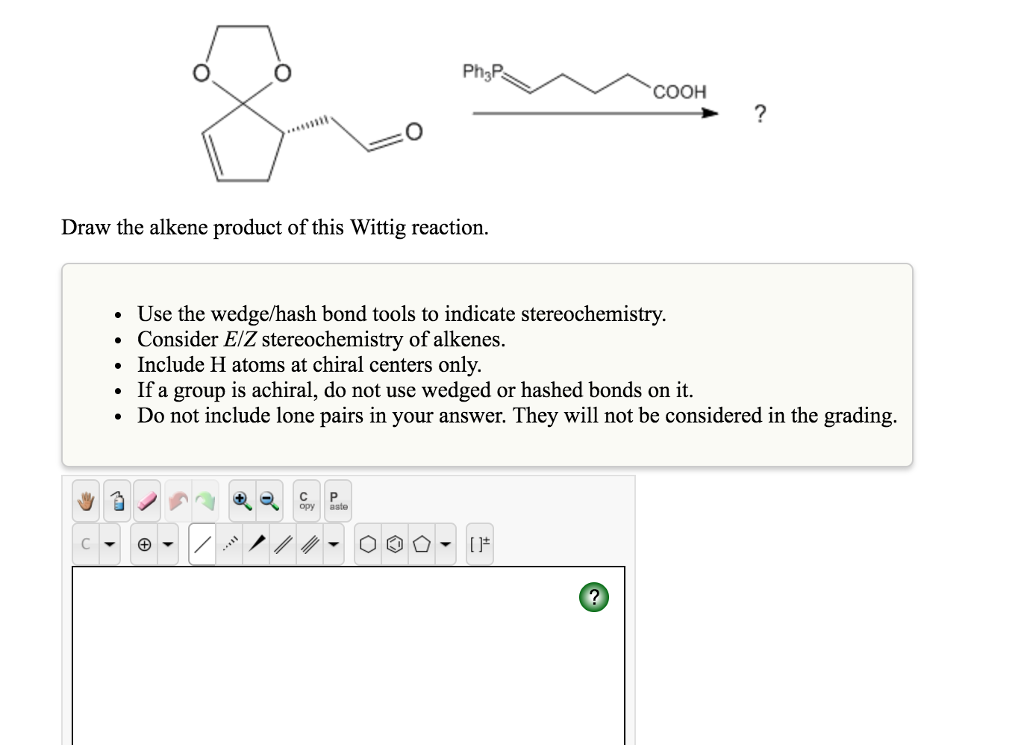
Solved Draw The Alkene Product Of This Wittig Reaction Chegg Com
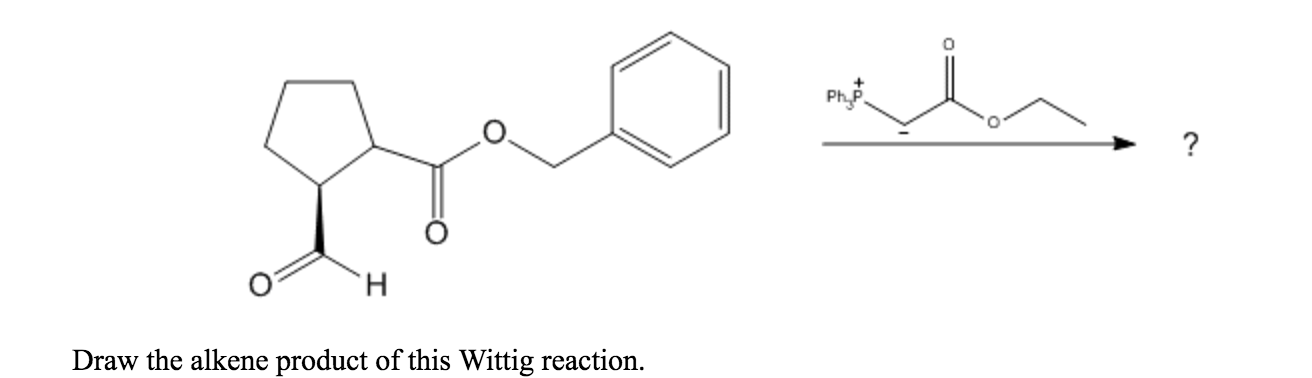
Solved 0 Draw The Alkene Product Of This Wittig Reaction Chegg Com
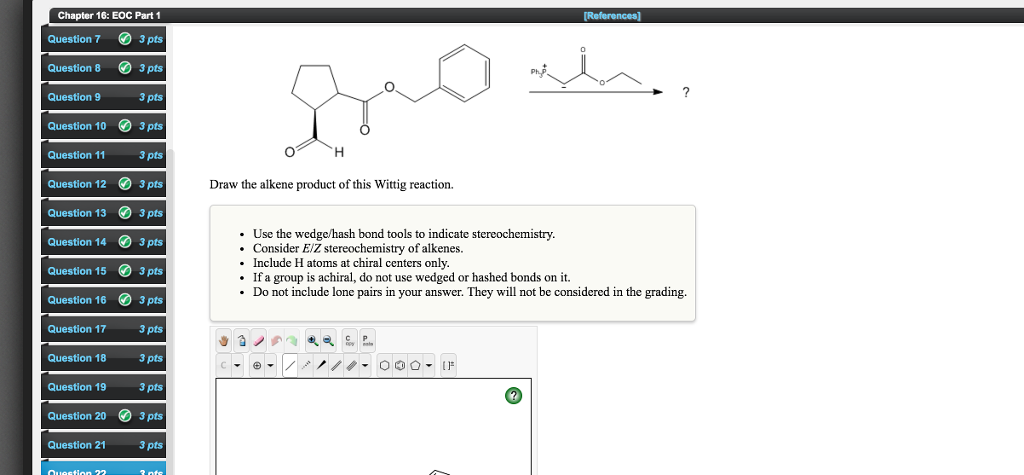
Solved Draw The Alkene Product Of This Wittig Reaction Use Chegg Com
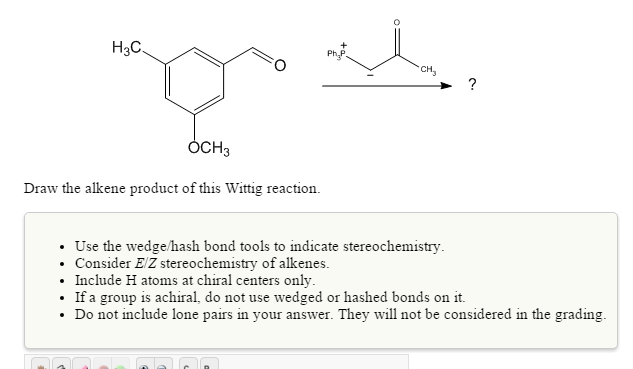
Solved Draw The Alkene Product Of This Wittig Reaction Use Chegg Com
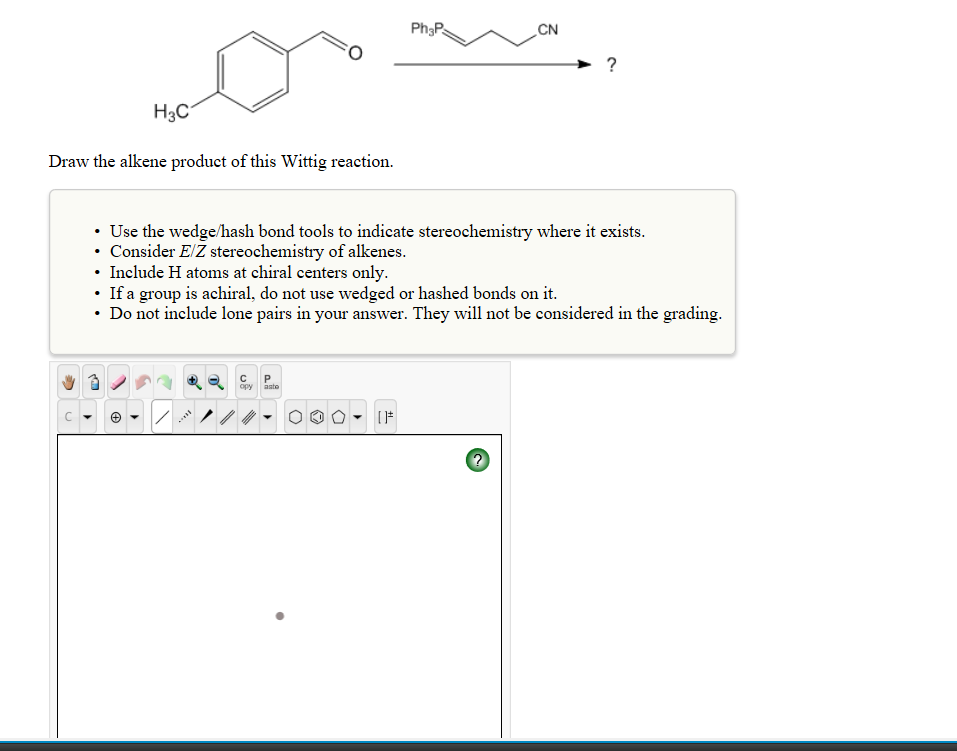
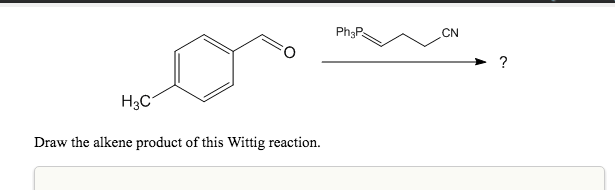
Solved Cn H3c Draw The Alkene Product Of This Wittig Chegg Com
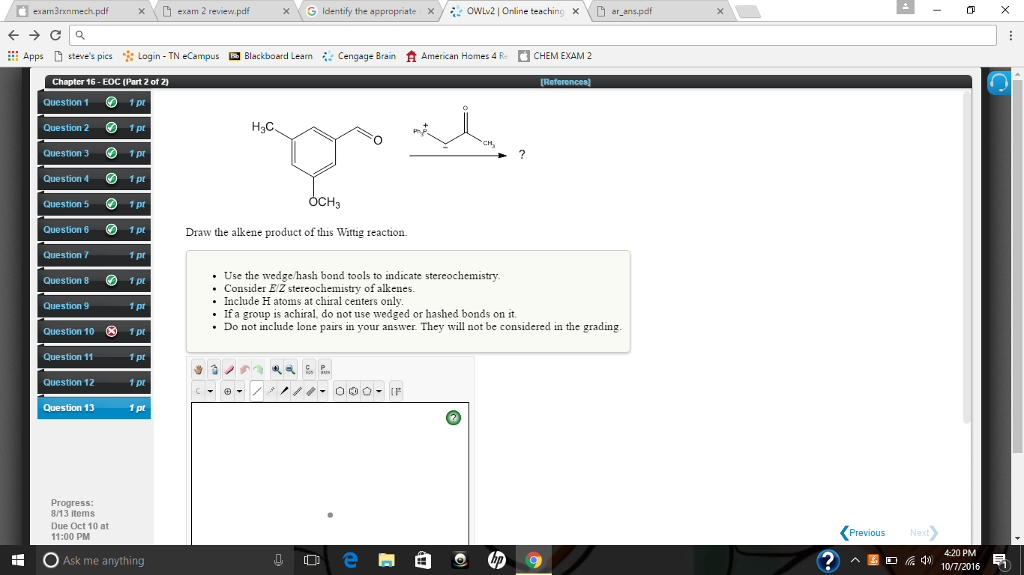
Solved Draw The Alkene Product Of This Wittig Reaction Chegg Com
0 comments
Post a Comment